Miranda Moore
RDA Lead Assistant, Beacon Dentistry
Dental Cycle Counting Guide: Accurate Inventory Without Disruptions
Keep chairs stocked and budgets steady. A practical guide to dental cycle counting—methods, cadences, and KPIs to maintain accurate inventory, minus the shutdowns.
Content
- What Is Cycle Counting in a Dental Practice
- Which Cycle Counting Methods Should a Dental Clinic Use?
- How Often Should You Cycle Count Each Category?
- How Do You Prepare the Practice for Reliable Cycle Counts?
- Step-by-Step Cycle Count Guide
- How Should Dental Practices Handle Expiration Dates, Lot Numbers, and Controlled Substances
- What Metrics Prove Your Cycle Counting Works, and How Do You Improve Over Time?
Dental inventory goes off the rails when counts are irregular and reorders are guesswork. Cycle counting fixes both: short, scheduled mini-counts that keep inventory accuracy high without freezing patient care—so critical items are always chairside and waste drops fast.
As a practical inventory management strategy, cycle counting inventory pairs light, routine inventory tracking with clear reorder points, so you’re counting inventory where it matters and maintaining accurate inventory levels even across multiple locations.
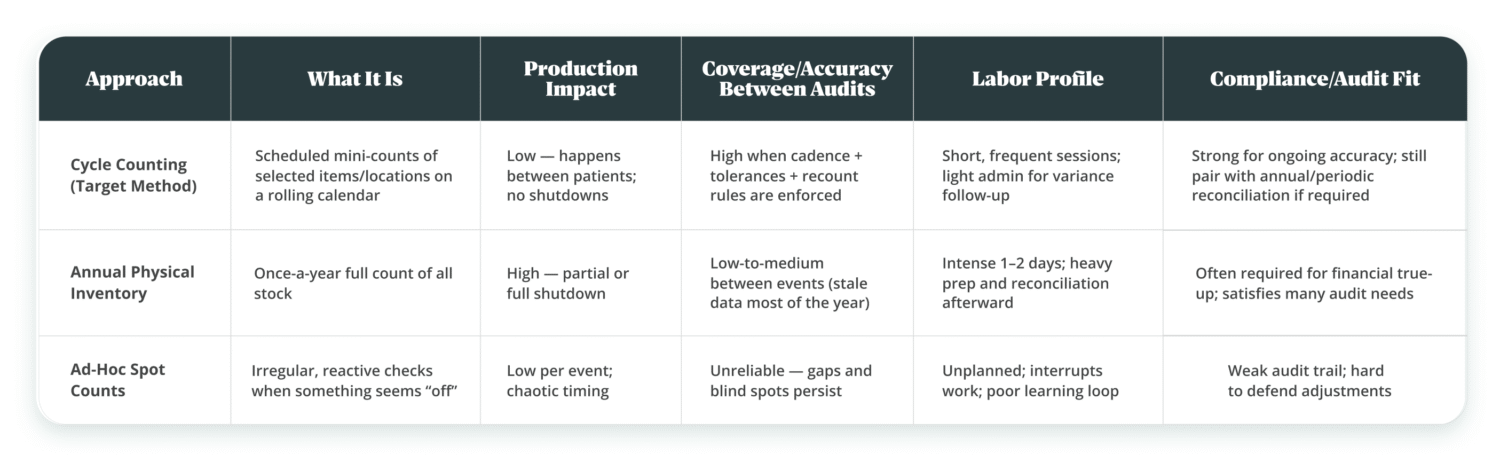
How Cycle Counting in Dental Practices Differs from Annual Inventory
Cycle counting is a rolling mini-inventory: you count a small, planned slice of stock on a schedule to keep accurate inventory levels during regular business operations, while an annual physical inventory count is a one-time full physical inventory count to true-up financials and satisfy audits.
In practice, an inventory cycle count runs in short intervals with your inventory management system (or inventory management software) scheduling inventory counts and reconciling each physical count; a cycle counting program based on simple inventory cycle counting methods (e.g., ABC) sits inside the broader inventory management process, while most clinics still keep an annual full physical count within their inventory control system for compliance.
Why does it matter for dental practices?
- Chairside availability: Mini-counts catch low stock before a procedure is delayed.
- Less waste: Frequent touchpoints surface hidden drawers and nearing-expiry items before they become write-offs.
- Minimal downtime: You count without closing ops; the cycle counting process fits between patients.
- Compliance still needs the big one: Many practices still run a yearly full physical inventory for financial reconciliation or board/insurer requirements.
Choosing cycle counts to protect production means accepting discipline: standardized SKUs, bin locations, and a repeatable counting method. The flip side of a once-a-year physical inventory is heavy disruption and “stale” accuracy for the other 364 days.
Cycle counting is like daily brushing and quick checks between patients; the annual physical inventory is your comprehensive exam and full-mouth series. Skip the daily habit, and the annual visit becomes expensive.
Which Cycle Counting Methods Should a Dental Clinic Use?
Most dental practices run a blended inventory cycle count program: ABC for focus, random samples to spot hidden issues in small catalogs, control-group repeats to watch stability, and event-based counts after receipts or big cases to validate inventory transactions quickly.
What Does ABC Cycle Counting Mean for Dental Supplies?
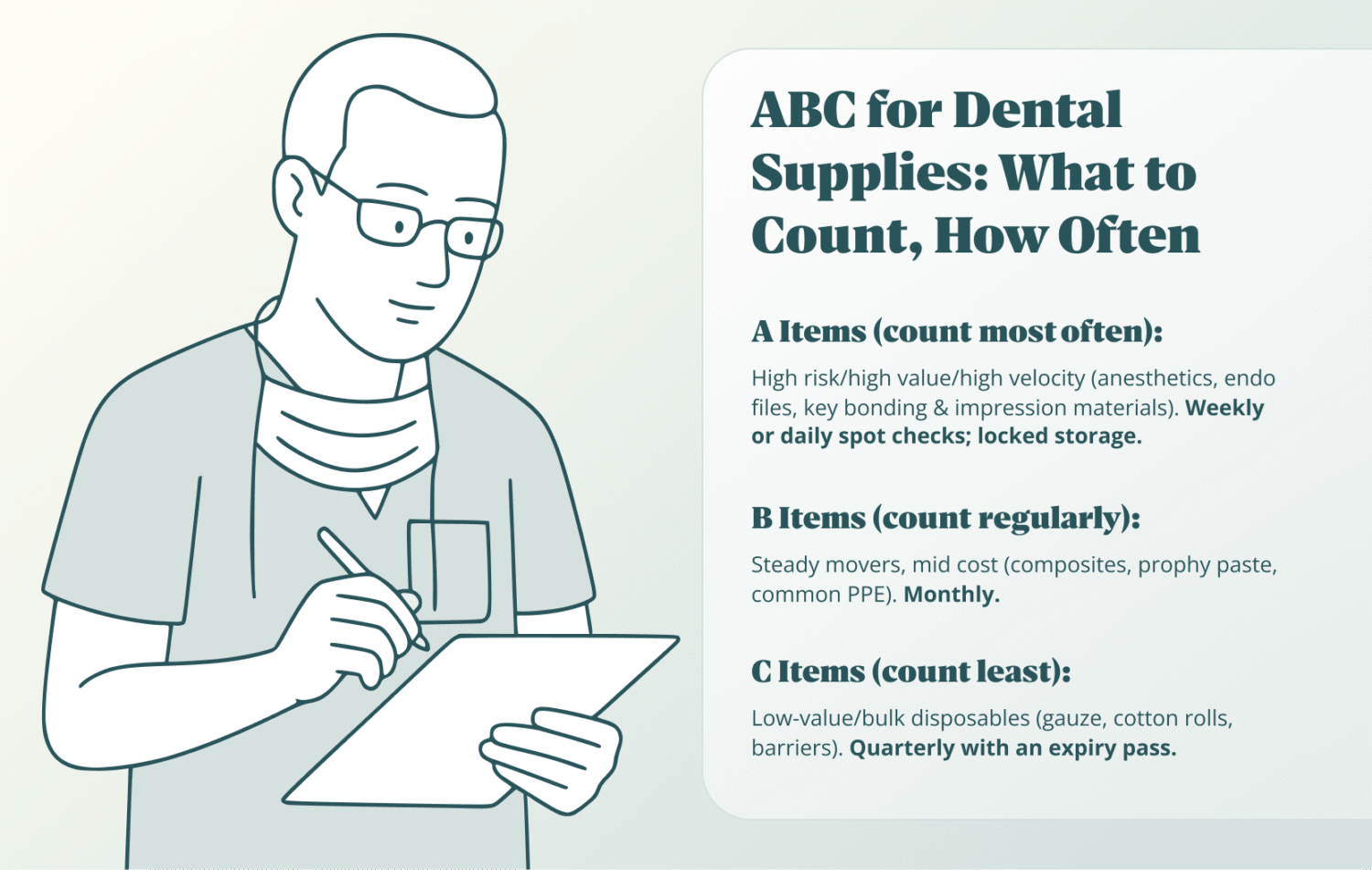
ABC prioritizes inventory items by clinical criticality, cost, and usage velocity, so A-items are counted most often, B monthly, and C periodically, putting attention where a stockout or variance hurts most. ABC is ER triage for supplies—treat the life-threatening issues first (A), then the stable cases (B), and review routine needs (C) on a calmer cadence.
How to map A/B/C for a dental practice:
- A (high cost / high clinical risk / high velocity): anesthetics, endo files, burs you burn through, etch, bonding agents, or impression materials.
- B (steady use / mid cost): composites, prophy paste, common barriers and PPE.
- C (low cost / bulk disposables): gauze, cotton rolls, patient napkins.
Steps:
- Pull last 30–90 days of use or purchases.
- Score each item across criticality, cost, and velocity usage velocity (how quickly you go through it).
- Assign frequencies (e.g., A weekly, B monthly, C quarterly).
- Publish a one-page schedule by room and central stock; owner + day + time block.
Choosing ABC for focus saves time on low-value counting, but you pay with upfront data cleanup (SKU names, units of measure) and the habit of keeping bin labels current.
When Is Random or Control-Group Counting the Better Choice?
Random samples are ideal when your catalog is small and you need a quick, unbiased pulse; control-group counts recheck the same subset routinely to monitor inventory records stability and your team’s counting consistency.
Use cases & compromises
- Random sample cycle counting: catches surprises (mis-shelved boxes, “ghost stock”). The upside is speed; the trade-off is uneven coverage of truly critical items unless you layer ABC.
- Control-group: pick 20–30 SKUs and recount them on a fixed interval to track inventory variance over time. You gain a clean signal on process health, but you spend time on the same items by design.
How to deploy without bloat:
- Keep the random sample small (10–15 items per session) and rotate rooms.
- For the control group, include a mix: 10 A-items, 10 B-items, and a couple of tricky C-items that often drift.
How Does Event-Based Counting Work After Receipts, Transfers, or Usage Spikes?
Event-based counts trigger right after high-risk moments—receiving deliveries, inter-room transfers, or unusual usage days (e.g., surgery blocks)—so errors are corrected while the trail is fresh.
Typical triggers for dental teams
- Receipts: verify quantities, units, and lots immediately at the door before items spread across operatories. Or have one room where all shipments and packages are delivered.
- Transfers: when moving stock between rooms, count the source and destination bins.
- Usage spikes: at the end of implant or endo days, spot-count key materials used in volume.
You get near-real-time accuracy where issues happen, but you must reserve small time windows and empower the lead assistant to pause other tasks for a clean check.
How Often Should You Cycle Count Each Category in a Dental Practice?
Set counting frequency by risk and velocity: quick weekly (or daily spot) checks for critical/high-value/high-turn items, monthly for steady movers, and monthly/quarterly for slow movers—baked into a calendar that rotates through operatories and the central stockroom without colliding with patient flow.
How Do You Set a Counting Calendar?
Build a weekly micro-cycle that assigns short blocks to rooms and the stockroom, scheduled outside peak hours, so counts happen fast and predictably.
- Mon: Op 1 (10 minutes before first patient), Op 2 (between patients before lunch).
- Tue: Central stock—A-items only (15 minutes), plus one shelf of B-items.
- Wed: Op 3 and Op 4 (same 10-minute windows).
- Thu: Finish B-shelf, quick pass in sterilization area for commonly pulled kits.
- Fri: Catch-up slot or small auditing inventory spot-check (random sample).
Assign an owner for each slot, keep the window tiny, and print/export a cycle count report so posting adjustments is routine, not ceremony.
How Frequently Should Critical/High-Value/High-Velocity Items Be Counted?
Count critical, high-value, and high-velocity items weekly—and add daily spot checks for open boxes in busy rooms—because even small inventory discrepancies create clinical downtime. Here’s an example of how it can be done:
- Weekly: anesthetic carpules, endo files, high-use burs, bonding agents with short open-bottle life.
- Daily spot check: open anesthetic boxes and burs in the rooms used that day (fast “open-box” tally).
- Exception logic: if an item shows repeated variance, escalate it to twice-weekly until stable.
More frequent checks reduce stockouts and emergency runs, but they require tight labeling and a 5–10-minute protected window; protect the window, or accuracy slides.
What About Slow-Movers and Rarely Used Supplies?
Slow-movers and rarely used items can be counted monthly or quarterly, but they need an explicit expiry check to prevent silent inventory write-offs.
- Monthly: low-use composites/shades, rarely used surgical items kept “just in case.”
- Quarterly: bulk disposables in overflow storage, seasonal items.
- Expiry pass: during these counts, pull anything within 90 days of expiration date forward to rooms scheduled to use it first, and quarantine anything that’s out of date.
Lower frequency saves time, but the reverse side is surprise expiries; pairing the slow-mover pass with a strict expiry review closes that gap.
How Do You Prepare the Practice for Reliable Cycle Counts?
Reliable cycle counts start before anyone touches a shelf: clean master data, labeled physical locations, clear roles, short pauses on movements, and a simple capture method keep inventory accuracy high and downtime low.
What Data Cleanup Is Required (SKU Names, Units of Measure, Categories)?
Standardize SKU names, units of measure, and categories so the team counts the same thing the same way—preventing synonym chaos and mismatched conversions that wreck inventory records.
- Normalize names: one canonical item name + aliases; remove duplicates that differ only by punctuation or vendor slang.
- Fix UOM ladders: define box → pack → each (and ml/g where relevant) with exact pack sizes; publish the only allowed conversions.
- Open-box rules: decide how to count partials (e.g., sleeves of 10 burs; carpules per blister); set rounding rules.
- Category taxonomy: Clinical > Restorative/Endo/Surgical/PPE; tag A/B/C class, expiry-sensitive, controlled, refrigerated.
- Fields to make mandatory: SKU, short name, UOM, pack size, location code, min/max or PAR, expiry flag.
- Guardrails against human error: lock free-text where possible; use dropdowns; archive retired items so they can’t be selected by mistake.
You invest time up front in the item master to avoid endless recounts later; choosing speed over standardization will introduce inventory errors that multiply with scale.
How Should You Label Bin Locations and Treatment Rooms for Counting?
Give every storage spot a unique, visible code so counters know exactly where to count items and where to post results.
- Schema: OP01-A3-BIN07 (Room-Shelf-Bin) for operatories; STK-S2-B4 for central stock.
- On-label data: location code, SKU short name, UOM, min/max (or PAR), and a small FEFO arrow if expiry-sensitive.
- Color cues: per room or category to speed recognition for assistants.
- Maps: print a one-page shelf map per room; keep the digital location list in your inventory management system.
- Geographic counting method: move left-to-right, top-to-bottom—never skip bins; mark each bin “counted” with a removable tag.
More labels mean setup time and a few reprints during reorgs, but you get faster counts and fewer misposted adjustments.
Who Should Count and When Should You Pause Movements?
Assign an inventory lead and a second counter, and pause issues/returns/transfers in the active zone so you’re not chasing moving targets.
- Roles: lead (assistant or office manager) runs the cycle counting process; second counter handles recount thresholds and spot checks.
- Windows: 10–15 minutes between patients; “one operatory at a time.”
- Pause scope: receiving, inter-room transfers, and returns for the zone; park them in a clearly labeled holding bin.
- Signage: “Count in Progress—Do Not Move Stock From This Location.”
- Exception log: list anything that couldn’t be paused (urgent use) to reconcile after.
Pausing inventory transactions for a few minutes slightly slows flow, but skipping the pause produces inaccurate counts and noisy variances.
Should You Use Barcodes and Mobile Scanning or Printed Count Sheets?
Use barcodes/mobile scanning for speed and fewer transcription errors; keep printed blind sheets as a resilient fallback when devices or Wi-Fi aren’t available. Automation increases accuracy and saves labor; the price is device upkeep and basic training. Pure paper is cheap to start, costly to sustain.
- Barcodes/mobile (preferred): fast entry, fewer inventory errors, easier lot capture, instant system count compare; needs devices, battery, and brief training.
- Printed blind sheets: work anywhere; no setup; but require later data entry, risk handwriting mistakes, and delay variance detection.
- Fallback flow: tally on paper → enter same day → second-person review for A-items → file the cycle count report.
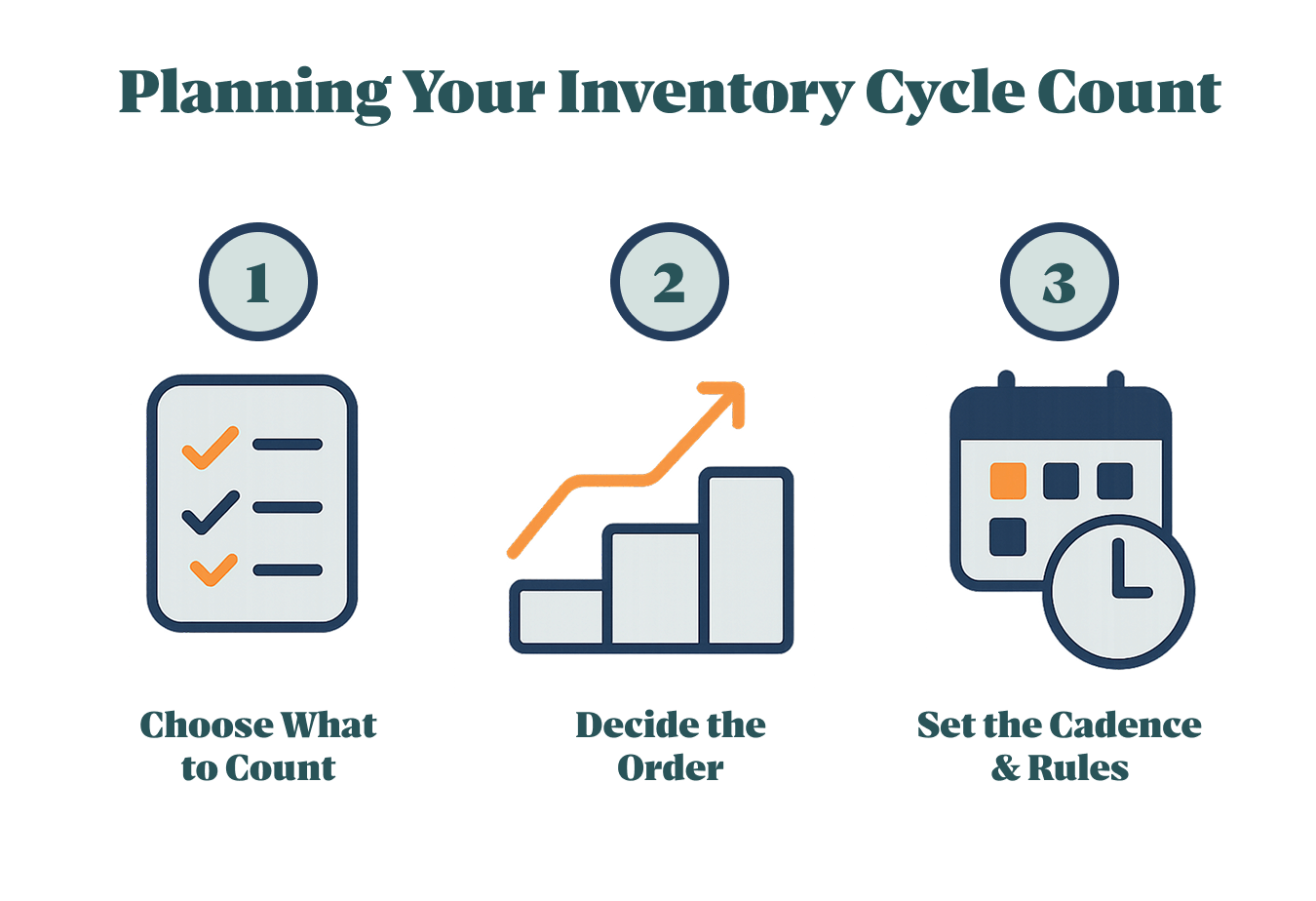
What Is the Step-by-Step Procedure for a Cycle Count?
A solid inventory cycle count follows four moves: freeze the scope, run a blind count, reconcile variances above tolerance, and post adjustments with audit notes—plus optional paths for clinic-hour counts and no-scanner days.
What Happens Before the Count?
Freeze the list of SKUs and locations, assign zones and people, and prep blind sheets or devices so counters see items—not the book numbers.
- Freeze scope: pick locations/SKUs; export the list; turn off on-screen quantities for true blind counts.
- Assign zones & owners: who counts, who recounts, who posts.
- Prep materials: printed sheets or mobile tasks; pens, removable “counted” tags; calculator for UOM conversions.
- Stage the zone: tidy bins; isolate returns; note any open cases to treat carefully.
- Tolerance bands: set recount thresholds (e.g., ±2 units for C-items; ±1 sleeve or ±2% value for A-items).
How Do You Execute the Count on the Floor?
Count blind by bin, handle open boxes deliberately, and trigger a recount when differences exceed thresholds—without reshuffling stock mid-count.
- Geographic pass: shelf by shelf, bin by bin; mark each bin as done.
- Open boxes: count inner packs or known sub-units (e.g., sleeves of 10); avoid guesswork.
- Kits/sets: count at the chosen level (whole kit or components) to avoid double-counting.
- Recount triggers: if variance crosses the band, a second counter recounts immediately.
- Contain movement: don’t “tidy” between bins; movement belongs after posting.
How Do You Reconcile Variances and Post Adjustments?
Compare physical counts to the system count, investigate deltas above tolerance, and post adjustments with clear reasons so future inventory reports tell a truthful story.
- Variance review: run the report; sort by $ impact and A/B class.
- Root causes: mis-shelved, wrong UOM, unlogged usage, receiving error, shrink.
- Second-counter: mandatory for A-items and any adjustment above a value threshold.
- Post & annotate: adjust with a reason code and a short note; attach photos if relevant.
- Feedback loop: if an item repeats, tighten its counting frequency, fix labels, or retrain on receiving.
Deeper investigation time reduces future noise; skipping it “saves minutes” but accumulates messy inventory variance and hidden inventory write-offs.
How Do You Run Counts During Clinic Hours Without Stopping Care?
Use micro-windows and a single-room scope: one operatory at a time, 10–15-minute blocks between patients, and a small cart with only the tools needed. Pre-print/export the room’s list; park a “count in progress” tag; pause pulls from that room only.
If a procedure needs an item mid-count, record the removal on the sheet and finish the bin before resuming.
How Do You Run Counts Without Barcodes or Mobile Devices?
Run printed blind sheets with clean handwriting, immediate same-day data entry, and double-signoff on high-value adjustments.
Think of the procedure like piloting a plane: pre-flight (prep), takeoff (blind count), mid-course correction (recounts), and landing (adjustments with a log). Skipping any checklist step risks the whole flight—even if the weather looks fine.
Use clear columns (SKU, location, UOM, count, notes); avoid free-text units. Enter results before day-end; second person reviews A-items; staple sheets to the adjustment export for an auditable trail.
How Should Dental Practices Handle Expiration Dates, Lot Numbers, and Controlled Substances During Cycle Counts?
Cycle counts should double as safety checks: every pass is a chance to surface near-expiry stock, preserve traceability through lot numbers, and verify that controlled substances are reconciled without gaps in the audit trail.
How Do You Verify Expiration Dates While Cycle Counting?
During the count, read the date on every expiry-sensitive item, record anything nearing expiry, and quarantine expired stock immediately so it can’t drift back into use.
- Record items with ≤90 days remaining (SKU, location, qty, expiry date) for priority use or return.
- Pull expired items into a clearly labeled quarantine bin; mark “Do Not Use.”
- Update the inventory records (adjust to zero in clinical bins; keep a paper/digital discard log with $ value).
- Move soon-to-expire lots forward in the FEFO flow; place them in rooms that will consume them fastest.
- If supplier policy allows, initiate a return/credit the same day to prevent inventory write-offs.
Tighter expiry checks reduce waste but add a few minutes to each pass; skipping them saves time now and costs dollars later.
How Do You Capture Lot/Serial Numbers Accurately?
Capture lot/serial at receipt and confirm it during counts in the same single source-of-truth field, tying each lot to a location (and to a patient when clinically required) so recall lookups are instant.
- Note lot/serial on the item record (not in free-text notes); scan where possible, or attach a photo to the cycle count report.
- Keep lot integrity by room: avoid mixing lots in the same bin unless the label shows “Lot A above / Lot B below.”
- When reconciling, verify that each lot in a room matches what the inventory management system shows; correct mismatches on the spot.
- For items used across multiple rooms, designate a “home” bin and document secondary locations to prevent cross-room mix-ups.
- After recalls, search by lot, quarantine matches, and attach the removal note to the inventory reports.
Think of lots like patient IDs for your supplies—if two charts get stapled together, the recall becomes a scavenger hunt.
How Do You Reconcile Controlled Substances Safely and Compliantly?
Run a separate mini-inventory with dual-count, locked storage, and signed logs; post adjustments with reason codes and keep the chain of custody unbroken.
- Maintain a separate controlled-substance log (daily or weekly, per policy); no commingling with general counts.
- Dual-count every adjustment (two signatures) and store in a locked cabinet with restricted access.
- Record lot/expiry with each movement; attach usage or wastage documentation to close the loop.
- Investigate any inventory variance immediately; escalate per policy (doctor/admin), and document actions taken.
- Archive records per board/insurer rules; exportable PDFs make auditing inventory faster.
Extra paperwork and two-person workflows slow you down, but the upside is legal safety and zero-tolerance visibility on shrink.
How Do You Count Surgical Kits and Sets Without Double-Counting?
Decide the counting unit up front—whole sealed set or individual components—and lock “open vs. sealed” rules to prevent counting the same items twice.
- Treat sealed kits as assemblies: count 1 kit = 1 unit; verify seal intact, no component counting.
- For opened kits, count components only; mark the kit “Open—Count by Components” until replenished and resealed.
- Exclude components already assigned to sealed kits from central stock counts (avoid double entries).
- Keep a simple BOM (bill of materials) for each kit so replenishment is quick and consistent.
- Post adjustments at the chosen level (kit vs. component) with a note to preserve traceability.
Counting at kit level is faster but hides component drift; counting components is precise but slower—choose based on risk.
What Metrics Prove Your Cycle Counting Works, and How Do You Improve Over Time?
Pick a few numbers that change behavior. Accuracy and variance trends show if counts are trustworthy; recount rules and root-cause notes show whether the process is learning or looping.
What Is Inventory Record Accuracy (IRA) and What Target Should You Set?
IRA is the share of items whose physical count matches the system within tolerance; most clinics aim for ≥97–99% on A-items and ≥95–97% overall.
- Item-based IRA: (Items within tolerance ÷ Items counted) × 100. Fast and clinic-friendly.
- Value-weighted IRA (optional): weigh by $ value to reflect financial impact; useful for audits.
- Quick compute: run the cycle count report, apply tolerances (e.g., ±1 unit for A, ±2% for B/C), and calculate both figures monthly.
Stricter tolerances drive better inventory accuracy but increase recount workload; balance by item class.
When Should You Trigger a Recount and How Do You Set Thresholds?
Trigger a recount when the difference exceeds a simple unit/percent band or a dollar threshold for high-value items; keep rules visible and consistent.
- A-items: ±1 unit or ±1% (whichever is tighter) → immediate second counter.
- B-items: ±2 units or ±2–3%.
- C-items: ±3–5 units or ±5%.
- Value gate: any variance ≥$X (set locally) triggers recount regardless of class.
- Enforce an “open-box rule” (e.g., count sleeves of 10) to avoid rounding noise.
Tight bands catch problems early but consume more counting resources; if bandwidth is thin, keep A strict and relax C.
How Do You Analyze Root Causes and Prevent Repeat Variances?
Treat variance as a symptom; tag a root cause and fix the upstream step so the same item doesn’t keep reappearing.
- Mislabeled bin / wrong location: relabel and update the map; short huddle with the team responsible.
- Wrong UOM or pack size: correct the master data; add UOM to the bin label.
- Receiving error: add two-person check on high-value shipments; scan lots at the door.
- Unlogged usage / skipped scans: retrain the room; add a weekly spot check.
- Open-box shrink: switch to inner-pack counting; move high-value items to supervised storage.
- Mis-picks / mix-ups across rooms: restrict mixed lots; set a “home” bin to anchor replenishment.
Investigation takes time now but reduces future noise; posting blind adjustments is faster and guarantees the same problem returns.
How Do Findings Adjust Future Counting Frequency and Zones?
Escalate “hot” items and relax stable zones; publish changes monthly so everyone sees the plan shift from punishment to prevention.
- Promote repeat-offender SKUs to twice-weekly or event-based counts until eight clean passes in a row.
- Add chronic offenders to the control-group subset for stability tracking.
- Create a “red zone” on the map for bins with recurrent drift; fix layout or access.
- After sustained stability, step frequencies down (e.g., A from weekly to biweekly) to reclaim time.
Dynamic schedules add management overhead, but they focus effort where it pays off.
Final Thoughts
You don’t need to rebuild your whole supply system to feel in control. Cycle counting works because it’s small, repeatable habits: count a shelf, label a bin, set one reorder point, and keep going. Tell your team what “done” looks like, give them a 10-minute window to do it, and celebrate zero-variance weeks the same way you celebrate on-time cases.
As accuracy climbs, anxiety drops. Operatories stay stocked, budgets stop lurching, and those Friday-night panic orders fade into memory. It’s not flashy, just quiet, boring reliability that lets you focus on patients instead of closets. Start tiny, keep it steady, and let the wins compound.
FAQ
Can Cycle Counts Fully Replace the Annual Physical Inventory?
Can Cycle Counts Fully Replace the Annual Physical Inventory?
Sometimes. If your cycle counting inventory program delivers 100% catalog coverage over the year, keeps audit trails (auditing inventory, inventory reports), and your auditor/insurer/state board accepts it, you can substitute a physical inventory count; otherwise run an annual full physical count to true-up the system count, financials, and inventory records for maximum inventory accuracy.
How Do You Treat Samples, Returns, and “Ghost Stock”?
How Do You Treat Samples, Returns, and “Ghost Stock”?
Segregate them. Use clearly labeled Samples, Returns/Quarantine, and Unknown/Ghost bins; nothing moves until logged in the inventory management system. For ghost stock, complete a location sweep, zero-out with a reason code, and attach a note/photo so the inventory variance/inventory discrepancies are explained and visible in your inventory reports—then resume counting inventory at normal inventory levels.
What Should Never Be Counted in These Routines?
What Should Never Be Counted in These Routines?
Exclude non-consumable fixed assets and instruments (handpieces, cassettes, equipment) from consumable inventory cycle counts; keep those in asset/sterile processing records. Clean scope is part of cycle counting best practices—your inventory control system and dental inventory management process should focus cycle counts on countable consumable inventory items only, using one consistent counting method.
How Do You Avoid Double-Counting Items Used in Multiple Rooms?
How Do You Avoid Double-Counting Items Used in Multiple Rooms?
Assign a “home” bin for each SKU and choose one reconciliation source (central vs operatory), then record transfers and reconcile once at that source of truth. Use clear location codes and a simple geographic counting method across the physical location(s)—especially in multiple locations—so inventory counts roll up to accurate counts without inflating the system count.
Join 1000+ dental professionals, shop from your favorite
suppliers, compare prices instantly, and save over $17,000/year
Try our platform free for 30 days.
Get the latest ZenOne updates and product launches in your inbox
Don't miss the latest news!
Receive exclusive offers and news straight to your inbox!
Continue reading
Let's discover how we can help you

Tiger Safarov
Hi, I'm Tiger, the CEO at ZenOne, and I'm happy to personally ensure your success with ZenOne. Send me your latest invoice or a statement for a Free Savings Analysis.
Ask me a question: